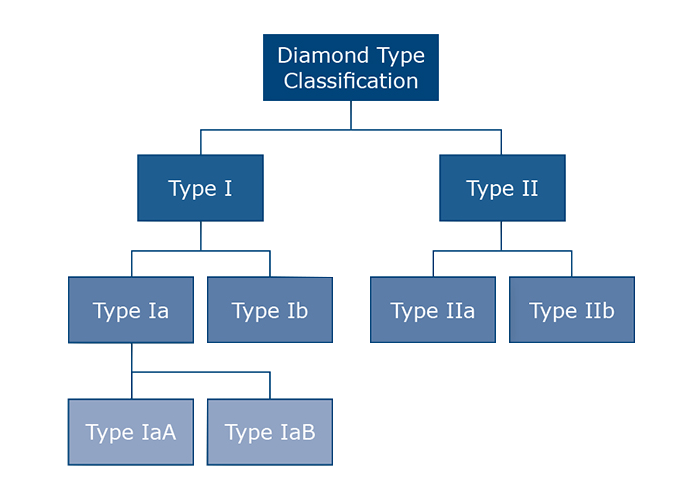
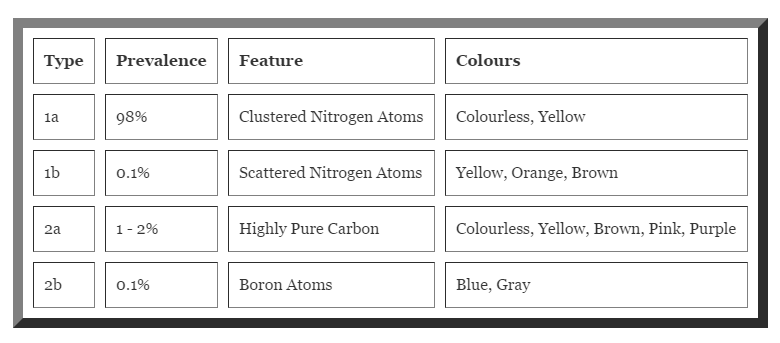
According to the chemical build of the stone, Diamonds are separated into four types: Type Ia, Type Ib, Type IIa and Type IIb. Diamonds are made of carbon, and are extremely pure, but in almost all diamonds there are tiny proportions of other elements, interspersed within the carbon as part of their crystal structure. These "impurities" are not what are known as inclusions, and are so small as to be invisible even under a very powerful microscope.
Interestingly, one stone can actually be classified as more than one specific type of diamond, as is the case in most stones in the market today.